REDEMPTION OF THE BEAST
The Carbon Cycle and the Demonization of CO2
by Randall W. Carlson (February 2017)
This essay and review of research into the carbon cycle and its effect upon the biosphere, more specifically upon the realm of plants and vegetation, is intended to provide a synopsis of evidence and information that is generally being neglected in mainstream discussions of Anthropogenic Global Warming (AGW) and to provide an enhanced and more realistic perspective on the effects of increasing concentrations of atmospheric carbon dioxide upon the world of nature and the world of society, which, of course, are inextricably linked.
I will begin the review by introducing two diagrams from relatively recent textbooks on Environmental Science that depict the Carbon Cycle throughout the various planetary sources, such as animal and plant respiration, volcanic eruptions etc. where carbon dioxide is released into the atmosphere, and sinks, such as the ocean and land plants, where CO2 is absorbed and taken out of the atmosphere. The basic numbers have not changed appreciably since the publication of these textbooks. The first chart is from Raven & Berg (2004) Environment 4/E; John Wiley & Sons, Inc. p. 106. Take note of the avenues of exchange from earth to atmosphere to ocean. The numbers are given in terms of “gigatons” of CO2 with a gigaton being equal to one billion metric tons. The reservoir of the atmosphere is the one that is currently the cause for greatest concern due to the greenhouse effect being intensified by increasing concentrations of CO2 resulting from the consumption of fossil fuels.
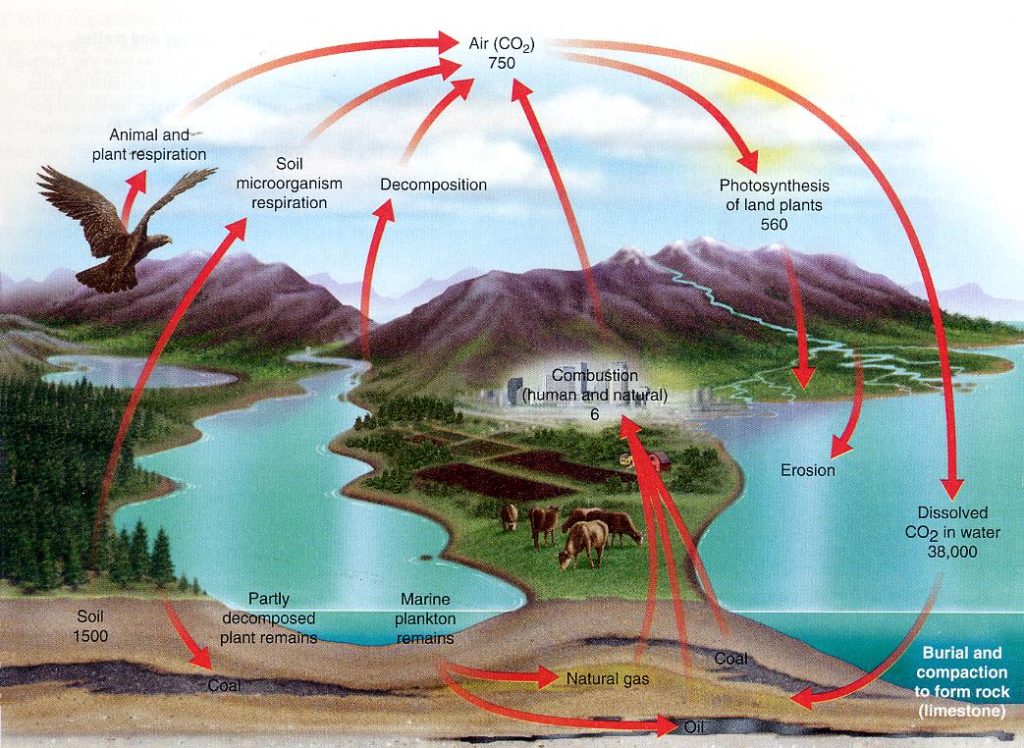
You will note that the amount of carbon dioxide in the atmosphere is given as 750 gigatons. You will also note that 560 gigatons are consumed in the process of photosynthesis by land plants. Take special note of the amount in the ocean: 38,000 gigatons, or 50 times the amount in the atmosphere. The soil at any time stores about 1500 gigatons. In the ocean the CO2 is taken up by a variety of marine organisms that have the ability to precipitate calcium carbonate (CaCO3) from seawater. This calcium carbonate forms the shells, or exoskeletons, of creatures such as scallops, bryozoans, foraminifera and coccolithophores. When these creatures expire, their shells drift down and consolidate on the ocean floor where they are eventually lithified under pressure into limestone, chalk and marble, to become part of the lithosphere or rocky crust of the Earth. This is the greatest of all the reservoirs of carbon dioxide storage. This chart does not show the estimated amount of CO2 stored in the lithosphere but it is enormous. Before going to the next graphic note that the amount generated through both human and natural combustion is 6 gigatons.
The next graphic also depicts the generalized global carbon cycle. It is reproduced from Botkin & Keller (2003) Environmental Science – Earth as a Living Planet; John Wiley & Sons, Inc. p. 63. It contains additional interesting details. Here fossil fuel burning accounts for 5.5 gigatons introduced into the atmosphere. This is one-half gigaton less than the preceding chart, presumably the one-half gigaton difference being the result of natural combustion and volcanism which is not included in this number. Storage in shallow ocean water is almost the same in both charts; fossil fuel deposits are shown to contain about 4000 gigatons of CO2 while the sedimentary rock reservoir contains upward of 100 million gigatons! This is truly a staggering amount of carbon dioxide, and all of it at one time passed through the global atmosphere before it was taken up by the oceans, converted into biogenic calcium carbonate, and locked up in the Earth’s crust. This is a clear implication that the ocean acts as a powerful pump, constantly extracting CO2 from the atmosphere and ultimately sequestering it into carbonate sedimentary rocks, where it remains for a very long time. The natural process of oceanic uptake, or absorption, is constantly depleting the Earth’s atmosphere of carbon dioxide, and if not replenished it would relatively quickly reduce the amount of CO2 to a concentration too low for effective photosynthesis.
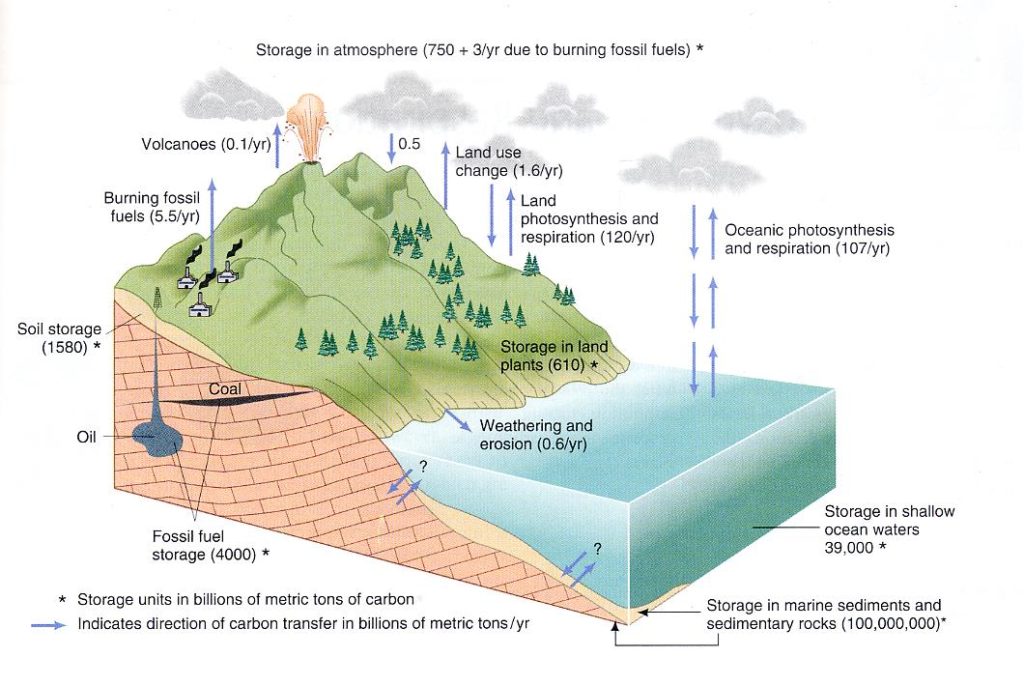
Finally, note that we have an additional interesting piece of information in the second graphic. The total amount of CO2 residing in the atmosphere is given as 750 gigatons (same as the first chart) along with an additional +3 gigatons per year due to burning of fossil fuels. Hopefully the reader is paying attention to the extent that they will see that this +3 gigatons is about half the amount initially introduced into the atmosphere due to fossil fuel combustion, given as 5.5 gigatons in this chart and 6 gigatons in the Raven & Berg chart. A substantial portion of the difference between CO2 released through combustion and the actual measured amount in the global atmosphere has been referred to as the “missing sink.”
The Missing Sink
The idea of a missing carbon dioxide sink began showing up in the scientific literature in the early-to-mid-1980s after several decades of air sampling and analysis. The program of atmospheric sampling dates back to 1968 when the National Oceanic and Atmospheric Administration (NOAA) began collecting air samples in Pyrex flasks, analyzing the contents and archiving the findings. Initially there were only two sampling sites but between 1971 and 1978 the number was expanded to 6 when NOAA launched the Geophysical Monitoring for Climatic Change (GMCC) program. In 1978 it was expanded again after the Department of Energy (DOE) started a program with the intention of looking at the longer term consequences of increased atmospheric CO2 concentrations. One goal of this research effort was the development of models to determine the partitioning of fossil fuel derived CO2 throughout the three principle global reservoirs: the oceans, the atmosphere and the biosphere. By 1982 a total of 23 sampling sites had been established. Samples at these sites were collected weekly and sent to the GMCC laboratory in Boulder Colorado for analysis. A full description of the sampling and analysis methodology can be found in [Komhyr, W. D. et al. (1985) Global Atmospheric CO2 Distribution and Variations From 1968-1982 NOAA/GMCC CO2 Flask Sample Data: Journal of Geophysical Research, Vol. 90, NO. D3, pp. 5567-5596, June 20.]
After five more years of sampling, three scientists from NOAA, NASA and Lamont-Doherty Geological Observatory, Columbia University performed an analysis on air samples collected from 1981 through 1987 and published their results in the journal Science in 1990. [See: Tans, Pieter P., Inez Y. Fung, & Taro Takahashi (1990) Observational Constraints of the Global Atmospheric CO2 Budget: Science, vol. 247, No. 4949, March 23, pp. 1431 – 1438] By this time it had become well established that the amount of CO2 in the atmosphere was increasing, however, as the authors of this paper comment: “After 30 years of measurements in the atmosphere and the oceans, the global atmospheric CO2 budget is still surprisingly uncertain.” They acknowledge the contribution of fossil fuel combustion on the observed increase in atmospheric concentrations but point out that the measured rise was only somewhat over half of the fossil fuel derived contribution. In estimating the fate of this missing carbon dioxide they point out that the uptake, or absorption, of CO2 attributed to the ocean was estimated to be between 26% and 44% of fossil CO2. Disinclined to believe the ocean was consuming that much they speculated that there must be a substantial, but as of yet, unidentified terrestrial sink. In conclusion the authors further comment that: “Our analysis thus suggests that there must be a terrestrial sink at temperate latitudes to balance the carbon budget . . . The mechanism of this C sink in unknown; its magnitude appears to be as large as 2.0 to 3.4 Gt of C per year . . .” Given that the total anthropogenic contribution of CO2 to the atmosphere is on the order of 6 Gt (gigatons) it is obvious that half of it is missing in action, so to speak.
In 1992, an article appeared in the Australian Journal of Botany by J.A. Taylor and J. Lloyd describing their analysis of global patterns of carbon sequestration. Their calculations and model runs indicated “the possibility that a significant net CO2 uptake, a CO2 ‘fertilization effect’, may be occurring in tropical rainforests, effectively accounting for much of the ‘missing sink.’ [See: Taylor, J. A. and J. Lloyd (1992) Sources and Sinks of Atmospheric CO2: Australian Journal of Botany, vol. 40, No. 5, pp. 407 – 418.] I will return to the question of the role of tropical rain forests in the uptake of carbon dioxide later in this review.
In 1995 a team of five scientists with NOAA and the Department of Geological Science at University of Colorado at Boulder, led by Phillippe Ciais, an atmospheric physicist with the Laboratoire des Sciences du Climat et de l’Environment, France, published the results of a study of weekly air samples taken from a global network of 43 sites by the Stable Isotope Laboratory at the Institute of Arctic and Alpine Research. These samples were measured for concentrations of Carbon 13, a stable isotope of carbon. In the words of the abstract to their paper, published in the journal Science, the concentrations and the isotopic ratios of carbon-13/carbon-12 in atmospheric carbon dioxide “can be used to quantify the net removal of carbon dioxide from the atmosphere by the oceans and terrestrial plants.” Without getting into the details of their procedure, the result of their analysis, as the abstract goes on to describe, was that: “A strong biospheric sink was found in the temperate latitudes of the Northern Hemisphere in 1992 and 1993, the magnitude of which is roughly half that of the global fossil fuel burning emissions for those years. The challenge now is to identify those processes that would cause the terrestrial biosphere to absorb carbon dioxide in such large quantities.” [For more see: Ciaia, P. et al. (1995) A Large Northern Hemisphere Terrestrial CO2 Sink Indicated by the 13C/12C Ratio of Atmospheric CO2: Science, vol. 269, Aug. 25, pp. 1098 – 1101] Yes, here was the challenge – it turns out that something in the terrestrial biosphere was absorbing large quantities of carbon dioxide and no one was quite sure what it was.
Studies continued on the terrestrial carbon cycle through the 1990s and by the turn of the millennium the problem of the missing carbon had still not been resolved although progress was being made in determining the proportions attributed to the ocean and to the land biosphere. A small sample of some of the work follows.
[Bender, Michael L., Mark Battle & Ralph F. Keeling (1998) The O2 Balance of the Atmosphere: A Tool for Studying the Fate of Fossil-Fuel CO2: Annual Review of Energy and Environment, Vol. 23, pp. 207-223]
“CO2 is added to the atmosphere by biomass burning and the combustion of fossil fuels. So added CO2 remains in the atmosphere. However, substantial amounts are taken up by the oceans and land biosphere, attenuating the atmospheric increase. . . Man is currently adding CO2 to the atmosphere at the rate of about 6.4 Gt C/yr by combusting fossil fuels and (to a small extent) by making concrete. We are adding another ~1 Gt C/yr by deforestation, mostly in the tropics. If all this CO2 remained in the atmosphere, the CO2 concentration of air would rise by about 3.5 ppm/year, much more than the observed increase of about 1.5 ppm/year. The difference between fossil-fuel input and accumulation, today as in the past, is attributable to the CO2 uptake by the oceans and by the growth of the land biosphere, as demonstrated by C. D. Keeling in a series of seminal publications.”
Here we learn several things. We learn that in addition to fossil fuel combustion, carbon dioxide is added to the atmosphere through biomass burning. We are informed that the rise in carbon dioxide based solely on anthropogenic emissions should be occurring at 3.5 parts per million per year. The measured increase, however, is only 1.5 parts per million each year. In this study it was found that only 42.8% of total anthropogenic emissions of CO2 was actually residing in the atmosphere. The remaining 57.2 % was, and is, being sequestered on land and in the oceans by natural processes. I should also point out that more recent studies suggest that cement production actually results in a net carbon dioxide sink rather than source, because, even though CO2 is released during cement production, concrete, it turns out, reabsorbs even more of it over the long term.
Other studies followed:
[Battle, M. et al. (2000) Global Carbon Sinks and Their Variability Inferred from Atmospheric O2 and δ13C: Science, Vol. 287, No. 5462, Mar. 31, pp. 2467-2470] “Between 1991 and 1997, combustion of fossil fuels added roughly 6.2 gigatons of carbon per year (GtC/year) to the atmosphere in the form of CO2. During this same period, the atmospheric burden of CO2 increased by only 2.8 GtC/year. The balance of the CO2 was taken up by the oceans and the land biosphere.”
In this report we are given a somewhat different perspective on the same situation as discussed in the Bender, Battle and Keeling article. There the total annual amounts are given as 6.2 gigatons and 2.8 gigatons, or, in other words, 55% percent of human emissions of carbon dioxide were missing, gone, no longer part of the atmosphere.
An article by Steven C. Wofsy, Abbott Lawrence Rotch Professor of Atmospheric and Environmental Science at Harvard University appeared in Science in 2001 [see: Wofsy, Steven C. (2001) Where Has All the Carbon Gone? Science, Vol. 292, June 22, pp. 2261 – 2263]
Wofsy writes:
“Emission rates of CO2 from combustion of fossil fuel have increased almost 40 percent in the past 20 years, but the amount of CO2 accumulating in the atmosphere has stayed the same, or even declined slightly. The reason for this discrepancy is that increasing amounts of anthropogenic CO2 are being removed by forests and other components of the biosphere. It is estimated that more than 2 billion metric tons of carbon – equivalent to 25 percent of the carbon emitted by fossil fuel combustion – are sequestered by forests each year.”
Here is a remarkable fact: One-quarter of the annual amount of fossil fuel sourced carbon dioxide was being consumed by forests alone. What effect could this process be having upon the forests of the world? I will come back to that question directly.
Liu, Zaihua, Wolfgang Dreybrodt, Haijing Wang (2010) A new direction in effective accounting for the atmospheric CO2 budget: Considering the combined action of carbonate dissolution, the global water cycle and photosynthetic uptake of DIC [dissolved inorganic carbon] by aquatic organisms: Earth Science Reviews, vol. 99, pp. 162-172
“One of the most important challenges in the science of global change is effective accounting of the global budget for atmospheric CO2. Anthropogenic activities have clearly altered the global carbon cycle and significant gaps exist in our understanding of this cycle. Roughly half of the CO2 emitted by burning fossil fuels remains in the atmosphere, and the other half is absorbed by the oceans and the terrestrial biosphere. The partitioning between these two sinks is the subject of considerable debate. Without robust accounting for the fate of CO2 leaving the atmosphere predictions of future CO2 concentrations will remain uncertain.”
Consider well the use of the term “uncertain” in this context. If the future CO2 concentrations are uncertain, how can any projections as to climatological effects be certain? As one learns more about the actual science involved in this debate about carbon dioxide driven climate change the more it becomes apparent that uncertainty is the preeminent trait of our present knowledge of global change on all levels, and the constant refrain that the science is settled are seen to be downright duplicitous.
Plants Love Carbon Dioxide
So, it is apparent that Nature has the ability to remove large amounts of carbon dioxide from the global atmosphere. It is this uptake of CO2 that removes at least half the amount that is being emitted through fossil fuel combustion. Whatever the exact distribution of CO2 into the various sinks it is clear that a substantial portion of it is being consumed by the biosphere in the process of photosynthesis. It is also apparent to many researchers that if not continuously replenished the ocean alone would relatively quickly sequester so much carbon dioxide from the atmosphere that it would severely affect photosynthetic processes. Many workers in the field have, over the years, commented on the paucity of CO2 in the atmosphere and the positive role it plays in biological processes. A couple of examples will serve to demonstrate the attitude about carbon dioxide before its demonization as the driver of global warming disaster. Near the end of the 19th Century T. C. Chamberlin, one of the most influential geologists of that era and founder of the Journal of Geology, wrote:
“The virtues of carbon dioxide are in inverse ratio to the sinister reputation which “a little knowledge” and a narrow homocentric point of view have given it. As a constituent of the atmosphere it is as necessary to the maintenance of life as oxygen because it is the food of plants and they in turn are the food of animals. Its peculiar competency to retain the heat of the sun renders it a decisive factor in the maintenance of that measurable constancy and geniality of temperature upon which the existence of life depends. It is a leading agency in the disintegration of crystalline rock and is a necessary factor in other geologic changes. It is an essential link in a chain of vital processes which involve all the constituents of the atmosphere.”
“. . . It is the least chemical constituent of a mixture that determines the amount of reaction. A loss of nitrogen or oxygen equal to .0003 of the atmosphere would doubtless be wholly inconsequential, while that amount of loss of carbon dioxide would be fatal to life and to many important geological processes.” [see: Chamberlin, T. C. (1898) The Influence of Great Epochs of Limestone Formation upon the Constitution of the Atmosphere: The Journal of Geology, vol. 6, No. 6, (Sept.—Oct.) pp. 609 – 621]
Here Chamberlin points out the fact that even a miniscule decline in the relative concentration of atmospheric CO2 would have serious consequences for global plant life. I would suggest that today we have a large group, who for various reasons – political, economic or philosophical, have chosen to take the “narrow homocentric point of view” by now attributing all climate change to the activities of mankind.
64 years after T.C. Chamberlin’s remarks on the importance of carbon dioxide for plants, soil scientist A. G. Norman, published an article in 1962 in the journal American Scientist. Norman had studied extensively into the relationship between photosynthesis and carbon dioxide, publishing dozens of papers from the 1930s through the 1960s. In his 1962 article entitled “The Uniqueness of Plants,” he commented on the vitally important role of CO2 in global biological processes. He addressed the paucity of atmospheric carbon dioxide relative to its importance as an essential plant nutrient.
“It is somewhat unfortunate that we have allowed the phrase “plant nutrients” to mean those inorganic elements that are essential for plant growth, because this causes us to forget the real substances from which the bulk of the stuff of plants is synthesized. The chemical engineer of whom we spoke earlier might be a little taken aback at being told that his only raw materials would be carbon dioxide and water . . . If one added, however, that the carbon dioxide would be available only at a concentration of 3 parts in 10,000 by volume, diluted with an excess of nitrogen and oxygen, he might appear troubled, because to him that would mean the handling of vast quantities of air to extract the carbon dioxide needed. The plant can do this. Let us not underestimate the stupendous quantities of carbon dioxide that are incorporated into vegetation. Figures of the order of 1.5 x 1010 tons [15 billion tons] annually have been quoted for incorporation into terrestrial plants (excluding the oceans). Perhaps one-thirtieth to one-fiftieth of this may be accounted for by economic crops, or plants used by man . . . even the last figure for crop plants exceeds several fold the yearly carbon dioxide output of all industrial operations on earth.”
Norman goes on to point out that:
“Perhaps it is easier to grasp figures based on an acre. To grow a good field of corn which will yield 100 bushels, about 20,000 pounds carbon dioxide is needed to provide about 5500 pounds for the organic structures of the crop. During the growing season, therefore, the corn plants on one acre must deplete an enormous volume of air to meet their needs for carbon dioxide. No less than 21,000 tons of air are needed to supply 20,000 pounds of carbon dioxide. This is a startling calculation because it points up the astonishing ability of plants to do what an engineer might describe as “processing” these many tons of air to recover and utilize about two and one-half tons of carbon.” [see: Norman, A. G. (1962) The Uniqueness of Plants: American Scientist, Vol. 50, No. 3, Sept., pp. 436 – 449]
The amount of carbon dioxide in the atmosphere since Norman wrote this article has increased to where it is now close to 4 parts in 10,000, by volume. This is still a miniscule amount when considered as a portion of the total atmosphere. If the amount of carbon dioxide in the atmosphere were to become diminished by a mere 2 parts out of 10,000 there would be serious detrimental repercussions to the process of photosynthesis, hence to the health of Earth’s plant life and to the entire biosphere as a consequence. One conclusion to be drawn from Norman’s comments is apparent: It takes a truly enormous amount of carbon dioxide to stimulate photosynthesis in the world’s vegetation. I will return to the question of the effects of reduced atmospheric concentrations later in this essay.
So, to summarize: Each year anthropogenic fossil fuel combustion releases roughly six gigatons of carbon dioxide into the atmosphere. Of this amount nature is rapidly consuming, or sequestering, about half, leaving only about three gigatons of residual human sourced CO2 remaining in the atmosphere.
The proportion of carbon dioxide in the atmosphere is measured in parts per million and at present stands at about 400 parts per million. (4 parts out of 10,000) Here is one way to look at the matter: For every one million molecules of air, (made up of primarily nitrogen and oxygen) there are 400 molecules of carbon dioxide. For comparison, there would be 209,500 molecules of oxygen, 780,900 of nitrogen, with about 9300 remaining for argon gas and a few other gases. In other words, the total mass of the atmosphere is over 2500 times greater than the carbon dioxide within it and some 625,000 times greater than the total amount of yearly contribution from fossil fuel consumption. Only 3 of these 753 gigatons, then, are the consequence of human activity in any one year period. The rest is the result of the natural activity of the carbon cycle, a difference of 250 to 1. Let’s take a moment to do a bit of revealing math. Dividing 400 by 1 million gives us the figure of .0004, the decimal fraction for the total amount of CO2 in the atmosphere. To see the amount of atmospheric CO2 resulting from human activity divide this number in turn by 250. The result is the decimal fraction .0000016. This is a very small number, not much greater than nothing at all when compared to the whole atmosphere. This number, then, represents the per annum incremental increase in atmospheric carbon dioxide concentrations presumed to be due to anthropogenic activity. Also, it is by no means certain that all of these 3 gigatons are completely the result of the burning of fossil fuel either.
Are we then supposed to accept the conclusion, without question or debate, that this miniscule additional amount of CO2 to the atmosphere each year is going to provoke such a horrendous planetary catastrophe that we must completely overhaul our energy system, in effect, according to some of the more extreme proposals, dismantle our industrial infrastructure altogether? I am not making an argument for any one kind of energy technology over another here, I am simply trying to put things into perspective. I am totally in favor of making the conversion to a post-carbon future. But if we seriously circumscribe the productivity of industrial society by over-regulating, over-taxing, or hamstringing vital industries in the energy sector we will actually delay the day when that conversion comes to pass. If we base our actions upon an exaggerated threat we will end up squandering our resources and natural capital on diversions and distractions from the truly critical concerns and problems that should head our list of priorities as an evolving, planetary civilization.
Studies of Plant Response to Carbon Dioxide Enrichment
Let’s return to the other side of the issue, the one that manages to be neglected in most mainstream discussions of climate change − the positive, yes positive, role of carbon dioxide in critical biological processes. As pointed out above, in the work of T. C. Chamberlin and A. G. Norman, the concentration of CO2 in the atmosphere is very low relative to its importance for the health of the biosphere because of its fundamental role in photosynthesis. Reduce the amount of atmospheric carbon dioxide by even a small amount and plant life suffers, and hence, the entire chain of life. In fact, the amount of reduction that would begin to have serious effects on plant life is a mere 2 parts out of 10 thousand. So, given that a small decrease in the amount of carbon dioxide in the global atmosphere would be detrimental to plant life, what about the biological and environmental effects of increasing the amount available to plants? As it turns out there exists an enormous body of research into this question and to that important matter we will now turn.
Studies going back decades have demonstrated conclusively that carbon dioxide is indispensable to a healthy biosphere, having remarkable effects on plants of all types. I could not possibly go into detail on the hundreds of studies performed throughout the 20th century making the case that enhanced carbon dioxide concentrations have a positive effect on the biosphere, but I will discuss enough of them to make the point.
Awareness of the beneficial botanical effects of carbon dioxide enhancement goes back centuries. In the early 1600s a citizen of Brussels, Jean-Baptiste van Helmont, planted a 5 pound willow tree in a container in which he placed 200 pounds of soil. For five years van Helmont carefully monitored the amount of water provided and at the end of 5 years the tree weighed over 169 pounds but the soil was diminished in weight by only 2 ounces. While some of this increased tree mass was the result of small quantities of mineral nutrients supplied by the soil, two horticultural scientists referencing the work of van Helmont, remarked in 1964, that “More forcibly it should remind us that the primary nutrient from which the bulk of the plant originates is the carbon dioxide from the atmosphere.” [See: Wittwer, S. H. and Wm. Robb (1964) Carbon Dioxide Enrichment of Greenhouse Atmospheres for Food Crop Production: Economic Botany, Vol. 18, No. 1 (Jan. – Mar.) pp. 34 – 56] I will return to the work of Wittwer and Robb directly.
The United States Department of Agriculture has, since its inception, published annual summaries of research and work relevant to agriculture. In the Experiment Station Record for 1904-1905, the Department provided a summary and translation of the experimental work of M. E. Demoussy, originally published in French in 1904. In the early 20th century Demoussy performed an experiment in France demonstrating the effects of carbon dioxide on a variety of plants. These experiments were an early demonstration of the benefits of carbon dioxide enrichment. The summary of the article in the Experimental Station Record was entitled “The growth of plants in atmospheres enriched in carbon dioxide.” The translation of Demoussy’s article reads as follows:
“The results of the author’s investigations in growing lettuce in an atmosphere enriched in carbon dioxide have been questioned as being too limited to permit of generalizations. The author has repeated his experiments with 16 species of plants, representing a wide range of families.
Duplicate series were grown, one in normal atmosphere containing about 3 parts of carbon dioxide in 10,000, and the other series in an atmosphere enriched daily by about five times the normal quantity of carbon dioxide. The experiments were continued for about 2 months, after which several portions of the plants were weighed. Among the species studied were coleus, lettuce, geraniums, centaury, mint, tobacco, balsam, fuchsias, etc. In all except the fuchsias there was a decided increase in the weight of the plants, the average amounting to over 60 percent increase. In addition the geraniums, begonias, mints, etc. were hastened in their flowering and flowered more abundantly in the atmosphere enriched in carbon dioxide than was the case with the plants grown under normal conditions.” [See: U. S. Dept. of Agriculture (1904-1905) Experiment Station Record, vol. XVI, p. 847. For the original article in French see Demoussy, M. E. (1904) Sur la vegétation dans des atmosphères riches en acide carbonique: Comptes Rendus de l’Académie des Sciences, vol. 139, No. 21, pp. 883-885]
Before moving on let’s ponder the results of this experiment over 100 years ago. The control group of plants was exposed to the ambient CO2 concentration, at that time measuring about 300 parts per million. The experimental group was exposed to 5 times that amount, or about 1500 parts per million. At the end of two months the average increase in the weights of the various species of plants exposed to CO2 enrichment as compared with control groups was over 60 percent. In other words the plants responded exuberantly to the increase in their food supply and thrived strikingly.
Wittwer and Robb, referenced above, discuss an early experiment that demonstrated the positive effects on plants of carbon dioxide enrichment, citing the work of M. B. Cummings and C. H. Jones in 1918, published in the Vermont Station Bulletin. The title of their report was “The aerial fertilization of plants with carbon dioxide.” Cummings and Jones initiated a series of trials in 1909 that extended over 7 years. Augmented supplies of carbon dioxide were supplied for 8 hours each day to plants growing in open boxes. Wittwer and Robb summarized the findings of Cummings and Jones: “Favorable yield increases were obtained with many crops. Yields of pods and seeds were enhanced in peas and beans. Potatoes produced more leaves and tubers. Large and heavier leaves were formed by foliage crops. Lettuce was very responsive. Flower crops produced blossoms earlier and in great profusion. Strawberries yield fruit far in excess of the controls.”
Wittwer and Robb also mention that extensive experimentation was being conducted in Europe as well. They note that some of these experiments showed that the yield of some food plants such as tomatoes and cucumbers was doubled, or even tripled, by the addition of CO2.
In the 1920s botanists with the Department of Plant Physiology and Pathology, Imperial College of Science and Technology, London, designed and constructed apparatus to improve standardization of testing, which prior to this time lacked sufficient controls to perform a reliable statistical analysis. [see: Bolas, B. D. & Henderson, F. Y. (1928) The Effect of Increased Atmospheric Carbon Dioxide on the Growth of Plants. 1: Annals of Botany, Vol. XLII, No. COLXVI, April, 1928. pp. 509 – 523]
The results of their initial testing were published in 1928. Reporting on some of their earliest efforts they explain, “In this apparatus, seedlings of white mustard grown for eighteen days during the early summer of 1923 yielded a dry-weight increase of 68 per cent. ±7.7 per cent. over the controls.” In regards to an extensive experiment performed on cucumber seeds they report that “in one of the experiments in the series now being performed the dry-weight ratio of the experimental plants to the control plants was 225 : 100 after fourteen days’ growth in air containing increased carbon dioxide.” They also comment on the work of German botanist H. Fischer published the year before: “Fischer in a recently published paper notes that plants grown in atmospheres rich in carbon dioxide are richer in chlorophyll than those grown in ordinary air. Increased greenness has been observed throughout the above series of experiments.” The authors’ final concluding remark describes the results on cucumber plants, “It is found that in all experiments the artificial enrichment of the air with carbon dioxide results in a large increase in the dry weight of cucumber plants as compared with the results obtained in normal air. An increase is evident within two or three days from the beginning of the experiment.”
These results are quite extraordinary. Contemplate the fact that the weight of the experimentally enriched plants was 2 ¼ times greater than the control plants.
Turning back to the experimental work of agronomists Wittwer and Robb, we will consider their 1964 study Carbon Dioxide Enrichment of Greenhouse Atmospheres for Food Crop Production. This report described a series of experiments conducted from 1962 to 1963 at the Plant Science Greenhouses at Michigan State University with a variety of crops grown in containers. In this study they remark that:
“The great demand of green plants for carbon dioxide, suggests that levels of this gas in the ambient air of a heavy crop stand, and particularly in enclosed greenhouse atmospheres, might become sufficiently depleted, that growth is reduced. Alternatively, carbon dioxide levels in plant growing atmospheres maintained substantially above the normal should favor growth.”
Two points should be emphasized here. First – the authors advise that, because of the great demand by plants for carbon dioxide, it is likely that in a greenhouse environment the atmosphere might quickly become depleted in this essential gas to the extent that it begins to impede plant growth, supporting the idea that ambient atmospheric amounts of CO2 are, in point of fact, precariously low relative to the requirements of the biosphere, a point I will come back to later in this essay. Second – plant growth could be stimulated through enrichment of carbon dioxide in the greenhouse atmosphere with potential economic benefit. The authors go on to describe the results of experiments in the tank culture of algae in which it was found that:
“. . . it is possible to increase, by as much as 50 to 100 percent or more, the rate of photosynthesis under natural conditions by ‘carbon dioxide fertilization’ . . . In fact, carefully controlled experiments, the results of which for many plant species have now accumulated, show that neither the normal carbon dioxide concentration of the air, nor this gas in water . . . is sufficient for saturating photosynthesis in moderate or strong light.” Here is a phrase we will encounter again: “carbon dioxide fertilization.”
The authors are here pointing out that normal carbon dioxide amounts in both air and water are not sufficient for maximizing photosynthesis. In other words, the plants could readily consume a great deal more carbon dioxide than they presently are under ambient conditions. There is an important implication here. From this perspective one could surmise that the world’s plants might actually, under current conditions, be suffering from a carbon dioxide deficit.
The following photographs from Wittwer and Robbs report dramatically demonstrate the remarkable effects of CO2 enrichment on a variety of plants. These show only a few of the many similar experimental results but are typical.

Figure 1. Tomatoes grown in a controlled environment with CO2 concentrations varying between 125 and 500 ppm. Present global atmospheric concentrations are right at 400 ppm. Compare with the next image. From Wittwer & Robb (1964)

Figure 2. Tomato plants grown in an environment with CO2 concentrations varying between 800 and 2000 ppm, that is, 2x and 5x the current atmospheric concentration. From Wittwer and Robb (1964).
In the image below Wittwer and Robb show results of carbon dioxide enrichment on several plant varieties after 30 days. The plants in the top row are leaf lettuce, second from the top is bibb lettuce, second from the bottom are tomato plants and the bottom is cucumber. The column on the left are plants grown in an environment with approximately 400 parts per million carbon dioxide, similar to the present concentration, and the column on the right shows plants grown with ambient CO2 concentrations of 1000 ppm. The difference in growth is obvious and impressive.


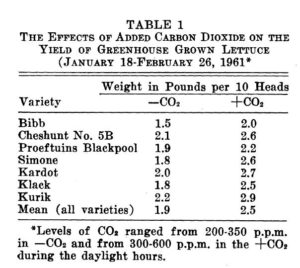
Table 1 below from Wittwer and Robb displays the effects on 8 varieties of lettuce. In every case the weight of the lettuce variety grown in an enriched environment was substantially greater.
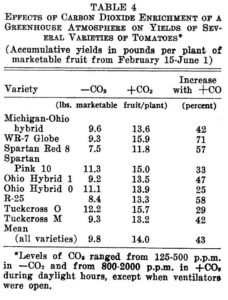
Experiments were also performed on a variety of tomatoes with the results shown below in Table 4 from Wittwer and Robb. Again, the remarkable effect on yield is impressive.
In summarizing their experiments, conducted at the Plant Science Greenhouses at Michigan State University, Wittwer and Robb discussed the effects on growth and development they observed during their experiments:
“The effects of above normal levels of carbon dioxide are even more pronounced on reproductive development than on vegetative growth. Tomato fruit yields were significantly increased . . . Fruit harvest was more consistent and less subject to fluctuating outdoor weather. Size and fruity quality were enhanced . . . Our data for cucumbers are supported by those of Daunicht where percent increases in fruit production were several fold greater than those for vegetative growth. . . More rapid growth rates, from carbon dioxide enrichment of a greenhouse atmosphere, have resulted in earlier maturity and higher yields of lettuce. Three crops of lettuce in place of two during fall, winter, and spring production are possible . . . Other benefits from growing crops in high levels of carbon dioxide in a greenhouse atmosphere have been reported. The percent dry matter is increased. The essential oil content has been enhanced. Rooting of cuttings was favored. Tomatoes had a higher vitamin C and sugar content, and plants were more resistant to some fungus and virus diseases and insects.”
In the conclusions to their report Wittwer and Robb confirm that their findings were similar to previous studies that found a substantial enhancement of plant growth. They made reference to Dutch botanist C. J. Briejer who published the results of his studies on plant responses to increased carbon dioxide concentrations in 1959 with the title Een verlaten goudmijn: koolzuurbemesting translated as “An abandoned gold mine: carbon dioxide fertilization”. Wittwer and Robb concur with the comment “Our findings lead us to agree.”
The authors conclude by emphasizing the benefits to be realized by commercial greenhouse operators with the employment of carbon dioxide enrichment:
“the grower of greenhouse vegetables has an unprecedented opportunity to strengthen his competitive position through substantial yield increases, a marked improvement of quality, and year around production by enrichment of greenhouse atmospheres with carbon dioxide . . . The production potential for greenhouse grown vegetable crops of the future is comparable to that which appeared a century ago when the agronomic benefits of chemical fertilizers were first realized. The ultimate possible rewards are so great that growers of greenhouse vegetables can begin immediately to use and to benefit from the results of the research findings herein reported . . .”
Is it possible that this “production potential” could be realized on the scale of the planetary biosphere? I will return to that question.
After 21 more years of study, one of these authors, the late Sylvan Wittwer, (who passed away in 2012 at the age of 95) reiterated the fact that there were benefits to the increase in atmospheric carbon dioxide. But then he went on to say something else very interesting. He, along with co-author Emeritus Professor of Biology, Boyd R. Strain pondered the effect of the increasing supply of CO2 on the planetary vegetation realm and speculated that:
“An increase in plant growth due to ‘fertilization’ of extra CO2 has not been measured, but a 5 to 10% increase may already have occurred. Current data indicate that plants growing at higher than normal CO2 levels are more tolerant of water, temperature, light, and atmospheric pollutant stresses. There are effects on carbon metabolism, plant growth and development, microbial activity, and terrestrial and aquatic plant communities.” [see: Wittwer, Sylvan H. & Boyd R. Strain (1985) Carbon dioxide levels in the biosphere: Effects on plant productivity: Critical Reviews in Plant Sciences, vol. 2, No. 3, pp. 171 – 198]
Here we see that by 1985 these scientists were speculating that there may have already occurred as much as a 5 to 10% increase in plant growth due to carbon dioxide fertilization, but at that time did not have sufficient data available to confidently make such a claim. We will come back to this question directly, and see that now, in 2017, we do have enough data to draw some conclusions.
Returning to the subject of carbon dioxide utilization in greenhouses, we will turn to a 1973 report by the Economic Research Service with the U. S. Department of Agriculture. In this report, titled “Global Review of Greenhouse Food Production,” the authors presented a brief exposition of early studies relating to the effects of controlled increases of carbon dioxide on plants. I will quote at length from that report:
“Carbon dioxide, along with water, is one of the two major ingredients in the process of photosynthesis. Below-normal levels of CO2, often found in unventilated greenhouses, can reduce the rate of photosynthesis, while above-normal levels can hasten photosynthetic activity.”
To repeat: The reason below-normal levels of carbon dioxide occur in unventilated greenhouses is that the plants will quickly consume all available CO2 as they grow and unless the supply is replenished it will then diminish to levels insufficient for continued photosynthesis, thereby stunting further plant growth. The Review goes on to discuss the upsurge in CO2 utilization in greenhouses in the 1960s due to development of safe and economical combustion sources of CO2 along with monitoring devices and plastic tubing for distribution and circulation. These innovations led to a major increase of testing and experimentation. The Review describes the situation in Europe at the time:
“The expansion first took place in Holland. It started in February 1961 when a grower used a small paraffin (kerosene) oil warming stove during the daytime hours on lettuce and obtained exceptional quality and weight: the effect was traced to CO2. Followup work at the Glasshouse Experimental Station in Naaldwijk showed outstanding results on lettuce and strawberries and good results on tomatoes, endive, spinach and radishes. The weight of the lettuce was increased and growth accelerated by 20 to 30 percent. During the 1962/63 season, the area of treated lettuce expanded into thousands of acres and 25 percent of the early greenhouse tomato growers used CO2. By 1972, the total area treated in Holland was about 7,000 acres . . .”
“The most recent wave of experimental work with CO2 in the United States began during the winter of 1961/62 at Michigan State University. Increases of 30 percent in lettuce yields were obtained; the following winter the figure rose to 70 percent . . . Increases in tomato yields ranged from 25 to 70 percent, depending on variety, and average 43 percent. CO2 proved to be especially effective in midwinter because crops in non-ventilated houses quickly utilize most of the available CO2 during the early morning hours and thereafter receive little benefit from sunlight.”[see: Dalrymple, Dana G. (1973) A Global Review of Greenhouse Food Production (Foreign Agricultural Economic Report No. 89) Washington D.C.]
A 1983 study on four plant species was conducted using transparent open-topped chambers placed in fields. Carbon dioxide was fed into the chambers by means of a ¾ horsepower fan and metered day and night. A high-pressure manifold allowed control of the ventilation airstream to generate three different CO2 concentrations. The tests were conducted by three botanists and the report describing the tests and their results appeared in the journal Science.
“The essential role of plants in the carbon cycle makes them a logical starting point for assessing the impact of elevated CO2 on living systems. Through photosynthesis, plants form the support system for the rest of the biosphere. Since carbon is a chief input in the food producing process, any appreciable response of plants to changing CO2 concentrations could have far-reaching implications.” [see: Rogers, Hugo H., Judith F. Thomas & Gail E. Bingham (1983) Response of Agronomic and Forest Species to Elevated Carbon Dioxide: Science, Vol. 220 (April 22) pp. 428 – 429
The four species upon which the tests were conducted were corn, soybeans, loblolly pine and sweetgum trees. The plants were grown in pots and exposed to the different levels of CO2 for a period of three months. All plant treatment protocols such as watering, fertilizing and light exposure were controlled and kept consistent. The carbon dioxide concentrations varied between 340 and 910 ppm. The results of the test was that:
“Growth was enhanced in all four species. Yield increased for the two crop species and wood volume increased for the tree species. . .”
And in addition:
“Plants growing in atmospheres containing 520 to 910 ppm CO2 did not undergo the wilting that we commonly observed for control plants on hot summer afternoons, when the rate of water uptake was exceeded by the rate of water loss. Wilting on hot afternoons inhibits leaf expansion and photosynthesis at a time when other environmental factors are most favorable for rapid carbon fixation. Thus a corn plant growing in an atmosphere with a high level of CO2 was able to continue fixing carbon and avoided wilting even though it had a greater leaf area.”
Some of the specific effects included:
“The leaves of soybeans, pine, and sweetgum thickened steadily as CO2 levels rose. At 910 ppm CO2, leaf thickness in these three species was 131, 110 and 121 percent of the control values, respectively. Thickness increased in all of the cell layers of pine and sweetgum leaves. In soybeans the greatest effect was the appearance of a well-developed third layer of palisade cells.”
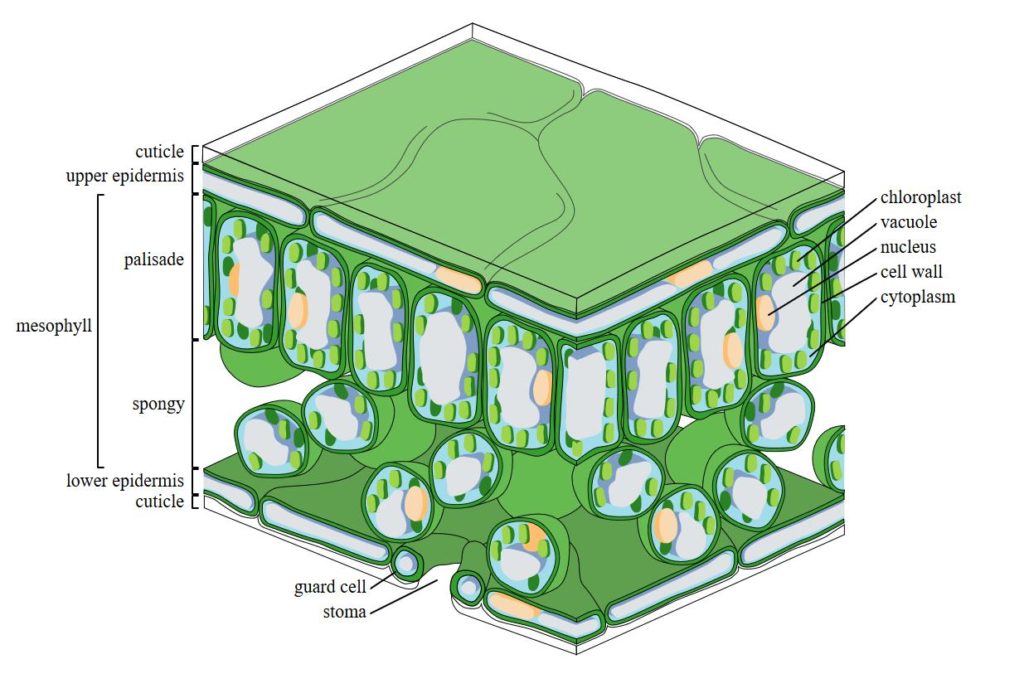
The appearance of a third layer of palisade cells is quite remarkable. Observe the image below from Wikileaks Common. The palisade is the vertically oriented row of cells containing the chloroplasts that is situated just below the upper epidermis of the plant. It is one of two layers comprising the mesophyll layer of the leaf which serves as the primary zone of photosynthesis due to the high concentration of chloroplasts. The main function of the palisade cells is the absorption of light by means of their chloroplasts, which are chlorophyll containing organelles, or sub-units, that absorb carbon dioxide and light energy during photosynthesis. The palisade cells containing the chloroplasts is typically one to two layers thick and is arranged in rows from one up to five in number. So now consider how remarkable it is that under a CO2 enhanced atmosphere the leaves of soybeans will construct a whole additional palisade layer of chloroplast containing cells allowing for enhanced light absorption.
In conclusion the authors state:
“These findings suggest positive growth responses to CO2 enrichment for the agronomic and forest species studied.”
To reiterate: By 1985 scientists were beginning to suspect the possibility that enhanced concentrations of CO2 may have been stimulating photosynthesis on a global level. But there is another point they make, one which reveals the remarkably important relationship of carbon dioxide to the health of plants, for by this time studies were revealing that in addition to growth stimulation, plants growing in a CO2 enriched atmosphere were more able to tolerate a variety of stresses, including water deprivation, light deprivation, temperature changes, and pollution, which I will discuss further.
Another important study was published in 1983 with a summary of research up to that date. The author was Bruce A. Kimball, a soil scientist with the U. S. Water Conservation Laboratory, Phoenix. His primary interest in this study was the effect of increasing CO2 concentration on agricultural yields. To that end he painstakingly compiled 430 observations on 37 different plant species grown under an enriched CO2 environment. The results of these 430 observations, conducted over 64 years, were published in 70 different reports evaluated by Kimball. He points out that most of the studies were performed in greenhouses and growth chambers. In regards to this circumstance he comments that “Open fields might respond less than greenhouses or growth chambers to increased CO2.” At this time in 1983 there had not been any serious effort to quantify the effects of carbon dioxide enrichment in an open field environment.
Extracting the data from these 70 reports Kimball included extensive appendices with his report. In reference to these Kimball remarks “As one scans the Appendices, it is apparent that CO2 enrichment has had an overwhelmingly positive effect on yield.” P. 780
“An overwhelmingly positive effect on yield.” This was the consistent message of 70 reports published over 64 years describing the results of 430 observations on the effect of a carbon dioxide enriched environment on plants. So, in quantitative terms, what does this overwhelmingly positive effect on yield translate into? Kimball elucidates:
“Plants are complex organisms, and undoubtedly there will be species differences and specific environmental differences affecting the amount by which the increased carbohydrate supply from increased CO2 is transformed into marketable yield. Certainly more data are needed for the major crops . . . Considering the variability inherent in such work, however, the large body of prior experimental data is sufficiently representative to provide a more reliable prediction of future CO2 effects . . . Thus, it appears from the analysis of prior data that agricultural yields will increase overall by about 33% with a doubling of the earth’s CO2 concentration.” [See: Kimball, B. A. (1983) Carbon Dioxide and Agricultural Yield: An Assemblage and Analysis of 430 Prior Observations; Agronomy Journal, Vol. 75, Sept.-Oct., pp. 779-788]
A 33% increase in agricultural yields would have a potent effect on the global economy. Food would become significantly more abundant. It is likely that Earth’s population will stabilize between 9 and 10 billion over the next half-century, assuming no natural extinction-level event or nuclear war occurs in the meantime. More yield per acre could be achieved, meaning more food from less land to feed the global population.
In the same year that he published Carbon Dioxide and Agricultural Yield, Kimball coauthored another valuable paper with Sherwood B. Idso. Idso has been associated with Arizona State University as Adjunct Professor in the Departments of Geology, Geography, Botany and Microbiology but his primary work was as Research Physicist with the U.S. Department of Agriculture at the Agricultural Research Service in Phoenix, Arizona. He has authored or co-authored over 500 scientific papers, has performed numerous experiments and has conducted extensive research into the biospheric and atmospheric effects of carbon dioxide. Professor Idso is still active and has become a provocative figure in the climate change controversies because of his willingness to acknowledge a beneficial aspect to carbon dioxide enrichment, along with his belief that increasing carbon dioxide is not going to have a dangerous effect on the climate. He is one of the foremost experts in the world on carbon dioxide’s role in nature. Yet, here is an example of a brilliant scientist who has been demonized by the global warming establishment for simply pointing out the truth about carbon dioxide’s beneficial role in life processes. His research is worth delving into further and directly I will review an important experiment he performed in the late 1980s.
Following upon Kimball’s earlier work, the authors summarize the conclusions derived from this comprehensive survey of experimental accomplishments up to that time:
“Probable effects of increasing global atmospheric CO2 concentration on crop yield, crop water use, and world climate are discussed. About 430 observations of the yields of 37 plant species grown with CO2 enrichment were extracted from the literature and analyzed. CO2 enrichment increased agricultural weight yields by 36%. Additional analysis of 81 experiments which had controlled CO2 concentrations showed that yields will probably increase by 33% with a doubling of atmospheric CO2 concentration. Another 46 observations of the effects of CO2 enrichment on transpiration were extracted and averaged. These data showed that a doubling of CO2 concentration could reduce transpiration by 34%, which combined with the yield increase, indicates that water use efficiency may double.” [see: Kimball B. A. & Idso, Sherwood B. (1983) Increasing Atmospheric CO2: Effects on Crop Yield, Water Use and Climate: Agricultural Water Management, vol. 7, pp. 55 – 72].
“Coupled with the increase in yield, the increases in water use efficiency are likely to be dramatic and probably will have a significant beneficial impact on irrigated and dry-land agriculture.” P. 61
Such findings in a depoliticized environment might be welcomed, but, as it is, almost no one outside of scientifically trained dissenters and “climate change deniers” even knows, or cares about this research, most especially is this true in the general public. It has also become apparent that efforts are being made to downplay the positive effects of carbon dioxide by arguing that there are limits to the fertilization effect upon plants. It is certainly true that when plants, particularly trees, reach maturity their need for carbon dioxide declines. It would be surprising if they did not consume less carbon dioxide in their senescence. The most vigorous uptake of carbon dioxide is during the growing phase of the plant’s life cycle. The fact that at some point there may be a limit to the botanical benefits of carbon dioxide enrichment does not negate the potent benefits that do accrue in the meantime.

